Immuno-Oncology Biotherapeutics
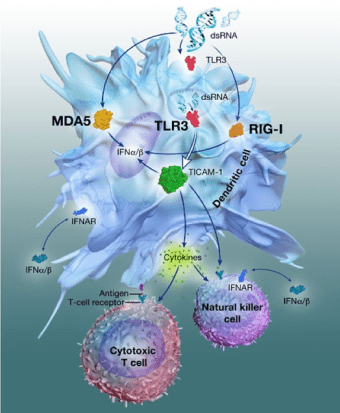
PIKA-based Biotherapeutics
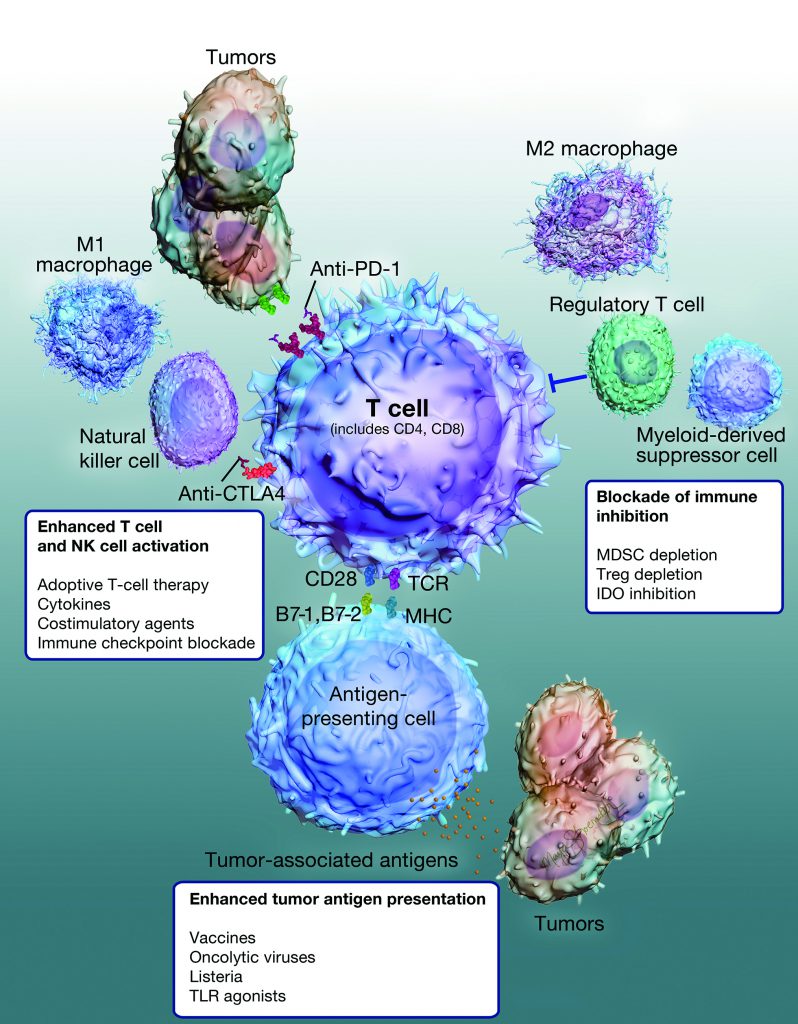
Disruption of Tumor Growth through Multiple Steps of Immune Activation
YS-ON-001
PIKA-enabled Anticancer Product Candidate
YS-ON-001 consists of a complex of protein and PIKA immunomodulating agent and is designed to reduce immunosuppressive effects within the tumor microenvironment and enhance antitumor immune responses. YS-ON-001 can be delivered via intramuscular, subcutaneous or intratumoral injection and has demonstrated a high rate of tumor growth inhibition in preclinical studies with models of breast, lung, liver, colorectal, prostate and other cancers.
In preclinical studies YS-ON-001 has demonstrated the ability to enhance macrophage phagocytosis, upregulate the activation of dendritic cells, natural killer cells and T cells, induce the production of multiple tumor-inhibitory cytokines and increase tumor cell apoptosis. Preclinical studies of the immune response in the tumor microenvironment have demonstrated that in addition to increasing CD4+ and CD8+ T-cell responses, YS-ON-001 can also significantly increase the proportion of natural killer and natural killer T cells. In several tumor models, administration of YS-ON-001 resulted in the reduction in the number of T regulatory cells and myeloid-derived suppressor cells. At the same time, M2 subtype macrophages of the tumor microenvironment reverse polarity to become M1 macrophages, enhancing the antitumor immune response.
The multiple mechanisms of action for YS-ON-001 suggest potential for development as a combination therapy with standard of care chemotherapies, targeted therapies, checkpoint inhibitors or with emerging cancer immunotherapies. YS-ON-001 has demonstrated antitumor efficacy as a standalone therapy and has demonstrated synergistic effects when combined with PD-1 checkpoint inhibition. Preclinical studies have also demonstrated enhanced anticancer activity of YS-ON-001 when combined with targeted therapies or cytotoxic agents.
YS-ON-001 has received Orphan Drug Designation from the U.S. FDA for development of the treatment for both hepatocellular cancer and pancreatic cancer. YS-ON-001 is currently in clinical development for advanced solid tumors.